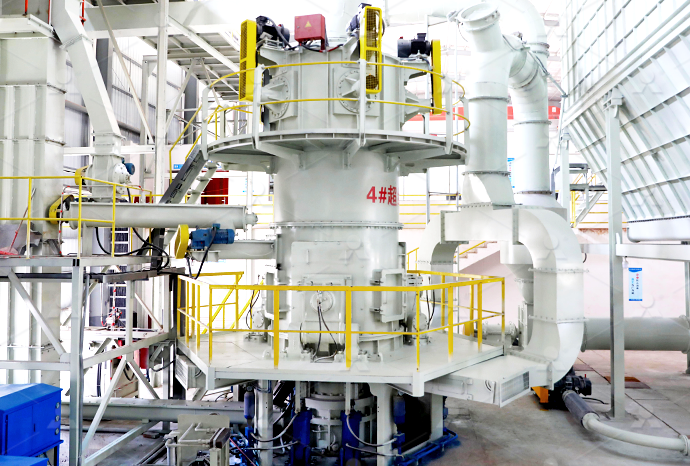
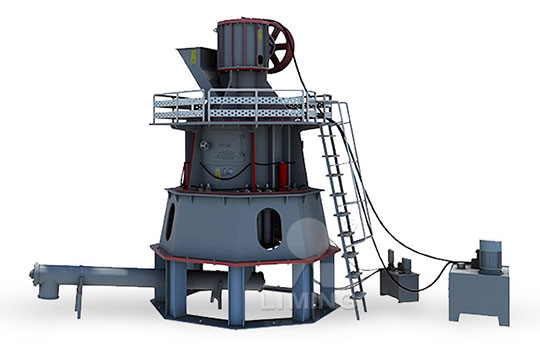
I want to set up a quicklime calcium oxide iron processing plant

Quicklime Lhoist
Crushed limestone is heated to around 1,100 degrees Celsius in kilns to produce quicklime The heating of limestone releases carbon dioxide, leaving calcium oxide (CaCO 3 produces CaO + CO 2 ) Quicklime has a wide range of uses, Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone (calcium Quick Lime Preparation, Properties and Uses Hebei Yayang 2024年4月16日 Here you will begin the process of turning your rock mixture into quicklime Heat your calcium carbonate directly on the flame until it becomes red hot Do this for about 23 How to Make Quicklime: 10 Steps (with Pictures) wikiHowQuick lime is produced by heating limestone (calcium carbonate) in a kiln to a temperature of about 900°C to 1000°C This process, known as calcinations, drives off carbon dioxide and Understanding Quick Lime: Types, Properties, and Applications
.jpg)
How to Make Quicklime: 10 Steps The Tech Edvocate
In this article, we will outline the 10 steps necessary for making quicklime 1 Gather materials: To make quicklime, you will need limestone (calcium carbonate) and a kiln or furnace to heat it at The production of high calcium quicklime begins with the selective mining of chemically suitable calcium carbonate Throughout the manufacturing process, we carefully monitor the process to Quicklime GraymontCalcium oxide, or quicklime, can be prepared via the thermal decomposition of seashells and limestone This process is generally referred to as calcination Take up a quiz on Quick limeQuicklime Preparation, Properties, and Applications with FAQsCalcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) There is a useful bit of video which shows the conversion of limestone, quicklime and slaked lime chemguide

High Calcium Quicklime Graymont
The production of high calcium quicklime begins with the selective mining of chemically suitable calcium carbonate typically containing less than 5% magnesium carbonate Throughout the Calcium oxide CaO Synonyms: Lime, Quicklime CAS Molecular Weight 5608 Browse Calcium oxide and related products at MerckCalcium oxide Lime, Quicklime MilliporeSigmaCalcium oxide CaO Synonyms: Lime, Quicklime CAS Molecular Weight 5608 Browse Calcium oxide and related products at MilliporeSigmaCalcium oxide Lime, Quicklime MilliporeSigma2020年3月20日 Properties Chemical Calcium oxide can be reacted, or slaked, with water, releasing large amounts of heat and producing calcium hydroxideIn open air, it reacts with water vapor and carbon dioxide, slowly converting to Calcium oxide Sciencemadness Wiki
.jpg)
Calcium Oxide Suppliers Quicklime Suppliers Calcium Oxide
We supply A grade Calcium Oxide to our all clients Calcium Oxide Uses Quicklime is used in the medical industry Quicklime is used in cement factories Calcium Oxide is used in paint factories Calcium Oxide is used in paper industries Burnt Lime is used in fertilizer Burnt lime is used in highgrade steel factories Quicklime is used for 2024年2月9日 Quicklime (Calcium Oxide) Heat Generator: Just 30g reacts with water to create up to 300°F of heat! Water Purifier: It can make water safer to drink with precise mixing (I wouldn’t use it as aQuicklime (Calcium Oxide) Heat Generator: Just 30g reactsI really don't want to make a kiln (to dry the hydrated lime) to be able to make a kiln and forge, among other things I need quicklime for a couple projects and it really is ridiculous that it is this hard to find considering there's probably 1/2 a pickup truck load for every truck of concrete madeWhere can I find quicklime/burntlime/calcium oxide/CaO to Through testing, the density of lime briquettes pressed by our lime briquetting machine can be up to 1922g/cm3 Lime Briquetting Plant Calcium Oxide Powder Briquetting Plant Please feel free to contact us if you have any questions about lime briquetting or Calcium Oxide / Lime Briquetting Plant
.jpg)
TIL the term "limelight" originated because when heated to
Theaters have a long and tragic history of claiming lives due to fires (google the Iroquois Theater Fire) Back in those days, when live flames were used for lighting and there weren't the kind of fire regulations we have now, incidents like these were not totally uncommon2022年8月11日 Calcium oxide was labelled the earliest chemical utilized by humans since it is an ionic material that people have used since the Middle Ages CaO Chemistry Calcium oxide’s chemistry: Calcium oxide has one cation and one anion The calcium cation with a valency of +2 and the oxygen anion with a valency of 2 make up the molecules of calcium Calcium Oxide (CaO) : Definition, Properties Uses TuritoI had this crazy idea that one could use calcium oxide (quicklime) we want the change in temperature to be from 20 degrees to 100 degrees q = (200) x (418) * (75) q = 62 700 Joules or 672 KJ Now if we look up the heat of the reaction that CaO releases when dissolved in water: CaO (s) + H2O (l) ⇌ Ca calcium oxide used to create exothermic reaction to heat foodFind stepbystep Chemistry solutions and the answer to the textbook question Lime is a term that indicates calcium oxide (CaO, also called quicklime) and calcium hydroxide [$\mathrm{Ca(OH)2}$, also called slaked lime] It is used in the steel industry to remove acidic impurities, in airpollution control to remove acidic oxides such as $\mathrm{SO2}$, and in Lime is a term that indicates calcium oxide (CaO, also calle Quizlet

A Trusted Name In The Trade Quick Lime
Quick Lime is produced through the calcination process, which involves heating limestone (calcium carbonate) to high temperatures (around 9001200°C) in a lime kiln This thermal decomposition process results in the release of carbon dioxide, leaving behind calcium oxide (Quick Lime) as the end product Conclusion:829 likes, 10 comments robbybenson on February 8, 2024: "Quicklime (Calcium Oxide) Heat Generator: Just 30g reacts with water to create up to 300°F of heat! Water Purifier: It can make water safer to drink with precise mixing (I wouldn’t use it as a sole purifier but it will aid in the process) Odor Fighter: Battles smells and pathogens in waste and compost Food Preserver: Rob Benson on Instagram: "Quicklime (Calcium Oxide) Heat Quicklime, CaO (calcium oxide), also called hical lime, is produced by the calcination of limestone, CaCO 3 (calcium carbonate) Crushed limestone is heated to around 1,100 degrees Celsius in kilns to produce quicklime The heating of limestone releases carbon dioxide, leaving calcium oxide (CaCO 3 produces CaO + CO 2)Quicklime Lhoist2024年10月11日 Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compoundIt is a white, caustic, alkaline, crystalline solid at room temperature The broadly used term "lime" connotes calciumcontaining inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominateCalcium oxide Alchetron, The Free Social Encyclopedia
.jpg)
Quicklime (Calcium Oxide) Heat Generator: Just 30g reacts
Quicklime (Calcium Oxide) Heat Generator: Just 30g reacts with water to create up to 300°F of heat! Water Purifier: It can make water safer to drink with precise mixing (I wouldn’t use it as aCalcium Oxide Quicklime Calcium oxide (chemical formula: CaO), also called quicklime or burnt lime, is a widely used chemical compound in our daily lives formed by ionic bonding between one calcium atom and one oxygen atom The white or grayishwhite crystalline solid, calcium oxide can be produced in large quantities by driving off carbon dioxide from calcium carbonateCalcium Oxide Quicklime Chemical Formula, UsesClick here:pointup2:to get an answer to your question :writinghand:take quick lime calcium oxide in a beaker containing water take a little of this Solve Guides Join / Login Use app Login Question Take quick lime (Calcium oxide) in a beaker containing waterTake quick lime (Calcium oxide) in a beaker containing water2020年8月25日 Set Up Now Enhancements you chose aren't available for this seller HiMedia GRM670500G Calcium Oxide Powder, Extra Pure, 500 g Synonyms: Lime, Quicklime CAS: Molecular Formula: CaO Molecular Weight: 5608 g/mol Test Specification 325 Mesh Calcium Oxide, 95+%, 25kg: Amazon: Industrial Scientific
.jpg)
Calcium oxide reacts vigorously with water to produce slaked
In the given reaction CaO(s) + H 2 O(l) → Ca(OH) 2 (aq) Calcium oxide Water Calcium hydroxide Part i) : lime (CaO) combines with water to form slaked lime Ca(OH) 2 (aq) Hence two substances are combined to form a single substance, therefore it is a combination reactionIn the process p ure limestone taken from the ground as calcium carbonate, then burnt in a kiln at approximately 850 degrees Celsius to create calcium oxide The calcium oxide is then crushed into 15mm granuals and under and is ready to be slaked Calcium oxide quicklime is regulary used in hot lime works and for building restoration usesCalbux 90 Quicklime for hotlime calcium oxide The Heritage When water (H 2 O) is added to quick lime (CaO), a lot of heat energy is produced along with Calcium hydroxide (Ca (OH) 2) It is an example of an exothermic reaction CaO + H 2 O → Ca ( OH ) 2 + heatWhat happens when water is added to quick lime calcium oxide?TIL: Before electricity, theatrical groups would heat up calcium oxide, also known as quicklime, for stage lighting This is where the expression “in the limelight” originates from People use to literally be in the limelightTIL: Before electricity, theatrical groups would heat up calcium oxide
.jpg)
limestone, quicklime and slaked lime chemguide
Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s) There is a useful bit of video which shows the conversion of calcium carbonate into calcium oxide and then calcium hydroxideATW, Inc, Santa Barbara, CA Manchak process uses quicklime to simultaneously stabilize and pasteurize sludge Quicklime, or a combination of quicklime and fly ash, is mixed with dewatered sludge at a predetermined rate in a confined space An instant exothermic reaction is created in the product wherein the pH is raised in excess of 12Using Lime to Beneficially Manage Wastewater Treatment Plant Calcium oxide (quicklime) reacts with water to produce calcium hydroxide (slaked lime) CaO (8) + H2O(0) + Ca(OH)2 (8) AH = 652 kJ/mol The heat released by this reaction is sufficient to ignite paper How much heat is released when 199 g of calcium oxide reacts? Enter your answer as a positive value AH =Solved Calcium oxide (quicklime) reacts with water to CheggSince calcium oxide rapidly hydrates to calcium hydroxide, and also in presence of air and carbon dioxide will recarbonate The final postclarification waste lime product mixture is in fact a calcium carbonate—chemically identical to limestone—rated at 8090% calcium carbonate equivalent, mixed with 4–10% organic matterSugar Lime and Calcium Oxide Organic Materials Review Institute
.jpg)
Want to buy Calcium Oxide Quicklime? Order it today at
What is Calcium Oxide – Quicklime? Calcium oxide, commonly known as quicklime, is an inorganic compound with the chemical formula CaOIt is a white, caustic, alkaline crystalline solid and is widely used in various industries Our Calcium Oxide is of high quality and ideal for professional applicationsQ Under what soil conditions do you think a farmer would treat the soil of his fields with quick lime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate)Calcium oxide is also called lime or quicklime BYJU'Sexpressed as calcium oxide, a strong caustic ingredient widely used in construction industry in the preparation of mortar and plasters It is also used for white washing of houses and building Iron and steel plants and foundries use lime as fluxing agent in considerable quantities Some drugs and pharmaceuticals, paper mills,33 PROFILE ON LIME PRODUCTIONCalcium oxide CaO Synonyms: Lime, Quicklime CAS Molecular Weight 5608 Browse Calcium oxide and related products at MerckCalcium oxide Lime, Quicklime MilliporeSigma
.jpg)
Calcium oxide Lime, Quicklime MilliporeSigma
Calcium oxide CaO Synonyms: Lime, Quicklime CAS Molecular Weight 5608 Browse Calcium oxide and related products at MilliporeSigma2020年3月20日 Properties Chemical Calcium oxide can be reacted, or slaked, with water, releasing large amounts of heat and producing calcium hydroxideIn open air, it reacts with water vapor and carbon dioxide, slowly converting to Calcium oxide Sciencemadness WikiWe supply A grade Calcium Oxide to our all clients Calcium Oxide Uses Quicklime is used in the medical industry Quicklime is used in cement factories Calcium Oxide is used in paint factories Calcium Oxide is used in paper industries Burnt Lime is used in fertilizer Burnt lime is used in highgrade steel factories Quicklime is used for Calcium Oxide Suppliers Quicklime Suppliers Calcium Oxide2024年2月9日 Quicklime (Calcium Oxide) Heat Generator: Just 30g reacts with water to create up to 300°F of heat! Water Purifier: It can make water safer to drink with precise mixing (I wouldn’t use it as aQuicklime (Calcium Oxide) Heat Generator: Just 30g reacts

Where can I find quicklime/burntlime/calcium oxide/CaO to
I really don't want to make a kiln (to dry the hydrated lime) to be able to make a kiln and forge, among other things I need quicklime for a couple projects and it really is ridiculous that it is this hard to find considering there's probably 1/2 a pickup truck load for every truck of concrete madeThrough testing, the density of lime briquettes pressed by our lime briquetting machine can be up to 1922g/cm3 Lime Briquetting Plant Calcium Oxide Powder Briquetting Plant Please feel free to contact us if you have any questions about lime briquetting or Calcium Oxide / Lime Briquetting PlantTheaters have a long and tragic history of claiming lives due to fires (google the Iroquois Theater Fire) Back in those days, when live flames were used for lighting and there weren't the kind of fire regulations we have now, incidents like these were not totally uncommonTIL the term "limelight" originated because when heated to2022年8月11日 Calcium oxide was labelled the earliest chemical utilized by humans since it is an ionic material that people have used since the Middle Ages CaO Chemistry Calcium oxide’s chemistry: Calcium oxide has one cation and one anion The calcium cation with a valency of +2 and the oxygen anion with a valency of 2 make up the molecules of calcium Calcium Oxide (CaO) : Definition, Properties Uses Turito

calcium oxide used to create exothermic reaction to heat food
I had this crazy idea that one could use calcium oxide (quicklime) we want the change in temperature to be from 20 degrees to 100 degrees q = (200) x (418) * (75) q = 62 700 Joules or 672 KJ Now if we look up the heat of the reaction that CaO releases when dissolved in water: CaO (s) + H2O (l) ⇌ Ca