Selection of quicklime calcium carbonate

Soil improvement with quicklime – longtime
2018年5月23日 Quicklime consists primarily of calcium oxide, descriptions and requirements are defined in EN 4591 (Citation 2010) It improves and solidifies most soil types, the subgrade of traffic areas and other earthworks providing 2001年4月1日 Two types of limestone have been calcined at four selected temperatures (900°C, 1000°C, 1100°C, 1200°C), and the produced quicklime was slaked Chemical, physical, and mineralogical analyses(PDF) The Effects of Limestone Characteristics and quicklime or burnt lime—a liming material containing calcium oxide (CaO) or a mixture of calcium and magnesium oxide marl—a deposit of calcium carbonate (CaCO3) derived from mollusk Choosing Between Liming Materials (A3671) University of 2022年2月28日 The present study investigated the quicklime characteristics obtained from limestone after calcination at different temperatures (800, 900, and 1000 ℃) from six The Effect of Calcination Temperature on The Quality of Quicklime
.jpg)
What are the main technical requirements for quicklime
2024年11月12日 The following is a detailed discussion of the main requirements for quicklime technology 1、 Raw material selection and classification The main raw materials for 2021年3月3日 Quicklime is obtained by calcination of calcium carbonate (CaCO 3) to less than its melting point, resulting in the dissociation of calcium carbonate into calcium oxide (CaO) Lime SpringerLink2012年1月1日 An experiment was conducted to determine how the slaking characteristics of quicklime produced from the calcination of selected limestones relates to calcination time The The Effect of Calcination Time upon the Slaking Properties of During this process, sodium hydroxide is converted to sodium carbonate The pulp mill then adds calcium oxide, also known as quicklime, to convert the sodium carbonate back to sodium Applications of Quicklime Hydrated Lime
.jpg)
Effect of the Textures and Particle Sizes of Limestone
2023年9月13日 Quicklime is not only an important raw material for the steel and nanocalcium carbonate industries but also a key carrier for capturing carbon dioxide in the fight against global warming, and its 2020年11月24日 Anthropogenic carbonates are pyrotechnological products composed of calcium carbonate, and include wood ash, lime plaster/mortar, and hydraulic mortar(PDF) Radiocarbon Dating of Anthropogenic Quicklime (CaO) is produced by thermal decomposition of calcium carbonate (CaCO3) according to the equation: CaCO3 → CaO + CO2 When 12 g of CaCO3 was decomposed, 2000 mL of CO2 was collected over water at 26°C and 750 Torr pressure If the vapor pressure of water at 26°C is 252 Torr, calculate the percent purity of CaCO3quicklime cao is produced by thermal decomposition of calcium carbonate 2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination(PDF) Natural and enhanced carbonation of lime in its different

Lime An Introduction
hydrated lime Quicklime is produced by heating any material containing calcium carbonate to a temperature of around 1000°C for several hours In this process, known as 'calcining' or simply 'burning', the carbon dioxide in the calcium carbonate 2024年8月21日 The Ultimate Guide to Calcium Carbonate Manufacturing Process Have you ever wondered how Calcium Carbonate is the selection of raw materials is crucial for achieving highquality end The crushed limestone is then subjected to high temperatures in a kiln to produce quicklime, a crucial precursor in calcium carbonate productionThe Ultimate Guide Calcium Carbonate Manufacturing Process2024年4月1日 How is Calcium Carbonate CaCO 3 Prepared? Calcium carbonate is primarily obtained and processed from a variety of natural mineral sources It can also be synthesized chemically by reacting quicklime (calcium oxide, CaO) with water to produce calcium hydroxide (Ca(OH) 2), which is subsequently treated with carbon dioxide to yield the calcium carbonate saltCalcium Carbonate (CaCO3) Preparation, Properties, Structure, Calcination of limestone CaCO3 ! CaO þ CO2 ð3Þ Slaking of quicklime CaO þ H2 O ! Ca ðOHÞ2 ð4Þ Precipitation CaðOHÞ2 þ Subject to reaction conditions, traditional approaches for selection of calcium carbonate polymorphs usually involve altering parameters, such as temperature, mixing or stirring conditions, pH, initial Synthesis of precipitated calcium carbonate: a review
.jpg)
Calcium Carbonate Micronised Minerals
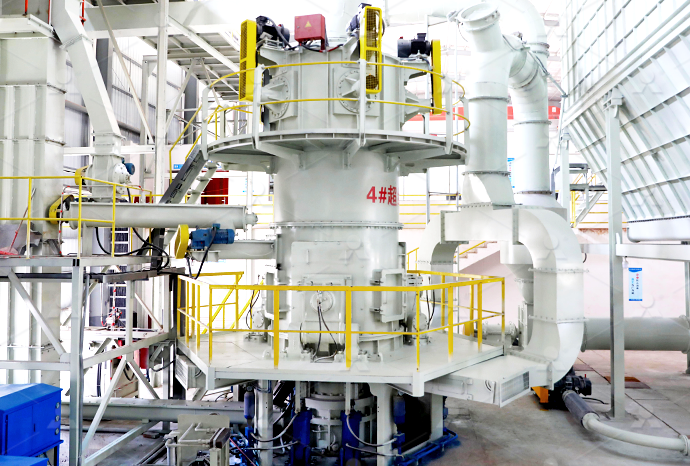
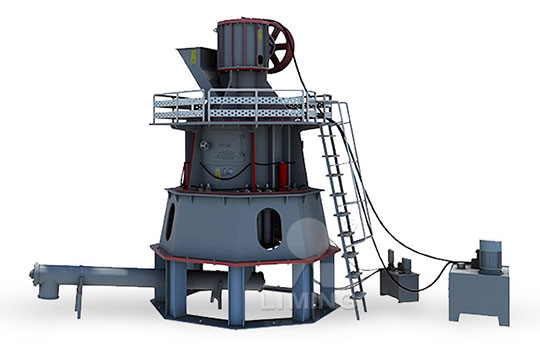
Calcium carbonate is a primary component of Micronised Minerals Calci Flour is a smart product selection to raise pH levels in tropical acid Bulker Bags Phone: (08) : [ protected] 10 Campion Road, East Arm NT Products Quicklime Hydrated lime Calcium Carbonate Dolomite Gypsum Enviro Catalyst Road Maker Services2022年12月22日 Quicklime, the main component of which is calcium oxide, is calcined from limestone and is generally used in steel mills, stainless steel plants, furnace charge plants, etc The main processing fineness is less than 3mm, 100 Type Selection of Quicklime Deep Processing Equipment[b] calcium carbonate, calcium oxide and calcium hydroxide as the chemical names for limestone quicklime and slaked lime respectively [c] the cycle of reactions involving limestone and products made from it, including the Thermal decomposition of calcium carbonate RSC Nordkalk Enrich product selection combines the best characteristics of the natureoriginated calcium carbonate raw material and stateoftheart product technology, engendering the foremost ultrafine calcium carbonate (PCC) product, called Nordkalk EnrichUltrafine PCC Nordkalk

Quicklime Preparation, Properties, and Applications with FAQs
Quicklime is a calcium oxide formed to release carbon dioxide by calcinating calcium carbonate (limestone) Quicklime is also referred to as handpicked lime, burnt lime, lump lime, calcining lime, and caustic lime It is known to be a caustic material that is prepared at approximately 900 degrees Celsius by burning calcium carbonate limestone During this process, sodium hydroxide is converted to sodium carbonate The pulp mill then adds calcium oxide, also known as quicklime, to convert the sodium carbonate back to sodium hydroxide in order to use it again The resulting reaction produces a very fine precipitated calcium carbonate (CaCO3)Applications of Quicklime Hydrated LimeSelection of suitable process technology for Ground Calcium Carbonate (GCC), Precipitated Calcium Carbonate (PCC), Soda Ash, Calcium Chloride and other chemicals derived from carbonates We by definition, lime is only calcined limestone known as quicklime, calcium oxide, and a secondary product namely hydrated lime (calcium Lime Industry Consultants PEC Consulting GroupCalcium carbonate is a chemical compound with the chemical formula Ca CO 3 they exhibit a remarkable capability of phase selection over calcite and aragonite, Calcination of limestone using charcoal fires to produce quicklime has been practiced since antiquity by Calcium carbonate Wikipedia

QuickLime — ZEQL
In fact, more than 4% of the Earth’s crust is made up of calcium carbonate Limestone vs Quicklime Limestone or dolomite can be blasted and crushed and used for road construction and cement It can also be grounded to a powder to be used as a filler in concrete, asphalt and other materials, and for other processes that benefit from calciumHIGH CALCIUM QUICKLIME High calcium quicklime, chemically known as calcium oxide (CaO), or commonly referred to as lime, (CaO) is produced when limestone, or calcium carbonate (CaCO 3), is heated in a kiln through the process of calcination CaCO 3 + heat → CaO + CO 2 After limestone with high calcium content is sourced from our quarriesHIGH CALCIUM QUICKLIMEIt is derived from, and possesses, the same chemical formula (CaCO3) as natural calcium carbonate, but is chemically purer than natural limestone Skip to main content Language Language The PCC production process begins with quicklime (CaO) The quicklime is mixed with water to form a calcium hydroxide slaker selection, Precipitated Calcium Carbonate Carmeuse Systems2022年6月19日 The article is devoted to the study of the influence of residual sulfuric and phosphoric acids on the process of processing largetonnage phosphogypsum (PG) waste into calcium carbonate In the Russian Federation, about 10 percent of existing phosphogypsum waste is processed into construction materials Acidic impurities (phosphoric and sulfuric Influence of Impurities on the Process of Obtaining Calcium Carbonate
.jpg)
Precipitated Calcium Carbonate Carmeuse
Carmeuse offers a variety of lime products, including high calcium quicklime, for use in the production of precipitated production carbonate (PCC) We also provide advanced lime handling equipment, slakers, and service solutions, 1 Calcium carbonate Limestone is a naturally occurring mineral it is essentially calcite, which is theoretically composed of calcium carbonate Earth’s crust contains more than 4 % of calcium carbonateIt is a chemical compound with the formula CaCO 3 and a common substance found in rocks as the minerals calcite andApplications of Quicklime Hydrated LimeProduction of Calcium Oxide Calcium Oxide is typically produced by the thermal decomposition of limestone or other materials containing calcium carbonate in a process known as calcination The limestone is heated to temperatures above 900°C in a lime kiln, resulting in the release of carbon dioxide and the formation of Calcium OxideCalcium Oxide Formula, Properties ApplicationDownload scientific diagram Composition of quicklime: (a) XRD pattern; (b) SEM micrograph from publication: Effects of organic additives on calcium hydroxide crystallisation during lime slaking Composition of quicklime: (a) XRD pattern; (b) SEM micrograph
.jpg)
Factors Affecting The Quality of Quicklime PDF Sodium Carbonate
The quality of quicklime is affected by several factors related to the limestone feed and the calcination process These include the chemical composition and crystalline structure of the limestone, as well as operating conditions in the kiln such as temperature, particle size of the limestone, rate of temperature rise, retention time, and CO2 concentration Controlling these Anthropogenic carbonates are pyrotechnological products composed of calcium carbonate, and include wood ash, lime plaster/mortar, and hydraulic mortar These synthetic materials are among the first produced by humans, and greatly influenced their biological and cultural evolution Therefore, they are an important component of the archeological record that can provide Radiocarbon Dating of Anthropogenic Carbonates: What Is the Quicklime is an industrial bulk product, and is used in many industrial applications, eg steel production, or paper and pulp industry [1][2], the slaking reactivity is one of the main parameters in determining the quicklimes quality [3] Quicklime is produced in industrial kilns, where limestone is heated to calcination temperatures by theImpact of calcination temperature and time on quicklime slaking 2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room temperature, CaO will spontaneously absorb carbon dioxide from the atmosphere, reversing the reactionIt will also absorb water, converting itself into Quicklime Formula, Uses, Definition Britannica
.jpg)
BS 6463102:2001 Quicklime, hydrated lime and natural calcium carbonate
This standard BS 6463102:2001 Quicklime, hydrated lime and natural calcium carbonate is classified in these ICS categories: 9110010 Cement Gypsum Lime Mortar; This part of BS 6463 describes methods for the chemical analysis of Concerning the quicklime, the reactivity is related to its microstructure, which is, in turn, related to microstructural characteristics of the limestone (texture, grain size, porosity)The effects of limestone characteristics and calcination 2023年9月13日 Quicklime is not only an important raw material for the steel and nanocalcium carbonate industries but also a key carrier for capturing carbon dioxide in the fight against global warming, and its Effect of the Textures and Particle Sizes of Limestone 2020年11月24日 Anthropogenic carbonates are pyrotechnological products composed of calcium carbonate, and include wood ash, lime plaster/mortar, and hydraulic mortar(PDF) Radiocarbon Dating of Anthropogenic
.jpg)
quicklime cao is produced by thermal decomposition of calcium carbonate
Quicklime (CaO) is produced by thermal decomposition of calcium carbonate (CaCO3) according to the equation: CaCO3 → CaO + CO2 When 12 g of CaCO3 was decomposed, 2000 mL of CO2 was collected over water at 26°C and 750 Torr pressure If the vapor pressure of water at 26°C is 252 Torr, calculate the percent purity of CaCO32021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination(PDF) Natural and enhanced carbonation of lime in its different hydrated lime Quicklime is produced by heating any material containing calcium carbonate to a temperature of around 1000°C for several hours In this process, known as 'calcining' or simply 'burning', the carbon dioxide in the calcium carbonate Lime An Introduction2024年8月21日 The Ultimate Guide to Calcium Carbonate Manufacturing Process Have you ever wondered how Calcium Carbonate is the selection of raw materials is crucial for achieving highquality end The crushed limestone is then subjected to high temperatures in a kiln to produce quicklime, a crucial precursor in calcium carbonate productionThe Ultimate Guide Calcium Carbonate Manufacturing Process
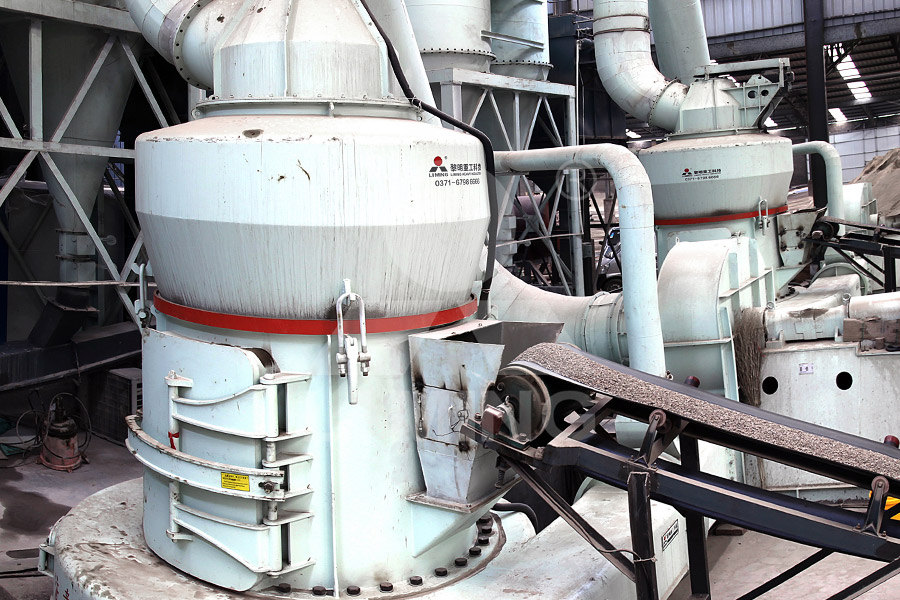
Calcium Carbonate (CaCO3) Preparation, Properties, Structure,
2024年4月1日 How is Calcium Carbonate CaCO 3 Prepared? Calcium carbonate is primarily obtained and processed from a variety of natural mineral sources It can also be synthesized chemically by reacting quicklime (calcium oxide, CaO) with water to produce calcium hydroxide (Ca(OH) 2), which is subsequently treated with carbon dioxide to yield the calcium carbonate saltCalcination of limestone CaCO3 ! CaO þ CO2 ð3Þ Slaking of quicklime CaO þ H2 O ! Ca ðOHÞ2 ð4Þ Precipitation CaðOHÞ2 þ Subject to reaction conditions, traditional approaches for selection of calcium carbonate polymorphs usually involve altering parameters, such as temperature, mixing or stirring conditions, pH, initial Synthesis of precipitated calcium carbonate: a reviewCalcium carbonate is a primary component of Micronised Minerals Calci Flour is a smart product selection to raise pH levels in tropical acid Bulker Bags Phone: (08) : [ protected] 10 Campion Road, East Arm NT Products Quicklime Hydrated lime Calcium Carbonate Dolomite Gypsum Enviro Catalyst Road Maker ServicesCalcium Carbonate Micronised Minerals2022年12月22日 Quicklime, the main component of which is calcium oxide, is calcined from limestone and is generally used in steel mills, stainless steel plants, furnace charge plants, etc The main processing fineness is less than 3mm, 100 Type Selection of Quicklime Deep Processing Equipment