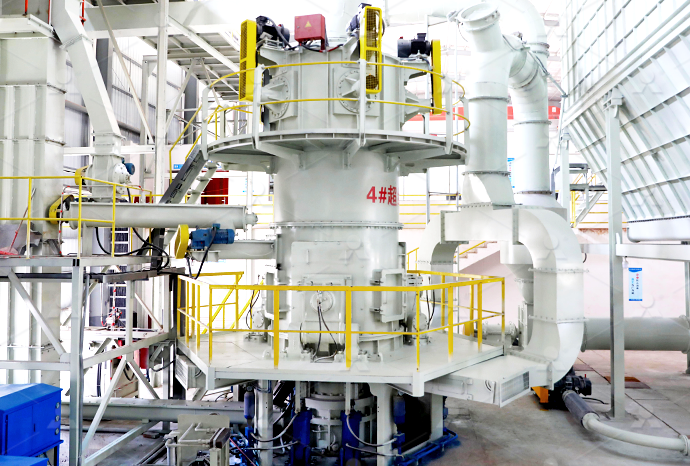
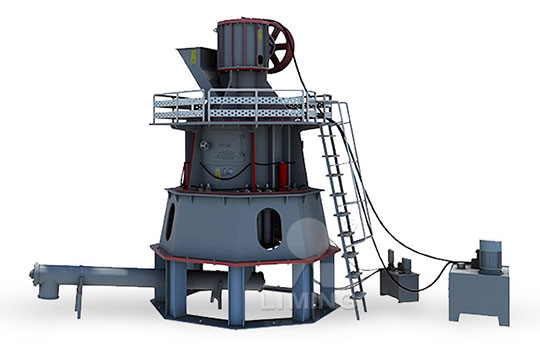
Quick lime calcium oxide high soil manufacturing process

Quick Lime (Calcium Oxide) manufacturers HTMC Group
HTMC Group is a prominent manufacturer and supplier of highquality quick lime, adhering to stringent quality control measures throughout the production process Their advanced lime kilns and calcination techniques ensure that the quick lime produced meets the highest industrial High calcium quicklime, or calcium oxide (CaO) is a white alkaline, crystalline solid widely used across many essential applications The production of high calcium quicklime begins with the selective mining of chemically suitable High Calcium Quicklime GraymontQuick lime is produced by heating limestone (calcium carbonate) in a kiln to a temperature of about 900°C to 1000°C This process, known as calcinations, drives off carbon dioxide and Understanding Quick Lime: Types, Properties, and ApplicationsAs a soil stabilizer the highly pure calcium oxide does its share as an additive for loamy soil Thanks to its high reactivity, this KFN quicklime extracts water from the soil in no time The Calcium oxide CaO Lime Kalkfabrik Netstal AG Firma

How is calcium oxide manufactured industrially?
2023年4月24日 Calcium oxide, also known as quicklime, is manufactured industrially by the thermal decomposition of calcium carbonate (limestone) in a lime kiln The process typically involves the following steps: Quarrying and Stabilization of problematic soils along with sediments and waste streams in environmental applications Quicklime can modify soils to minimize, if not eliminate, shrink/swell potential, Quicklime (Calcium Oxide) Mintek ResourcesHigh calcium quicklime (CaO) is produced when limestone, or calcium carbonate (CaCO 3), is heated in a kiln through the process of calcination CaCo 3 + heat > Cao = CO 2 After limestone with high calcium content is sourced from our High Calcium Quicklime CarmeuseCalcium oxide that survives processing without reacting in building products, such as cement, is called free lime [5] Quicklime is relatively inexpensive Both it and the chemical derivative calcium hydroxide (of which quicklime is the base Calcium oxide Wikipedia
.jpg)
QUICKLIME Calcium Oxide Ciaries
Ciaries’ calcium oxide, also known as quicklime, is valued for its reactivity and purity We ensure the quality and homogeneity of our product by optimizing the production process using the Throughout the manufacturing process, we carefully monitor the process to produce a high calcium quicklime that is highly reactive and has suitable particle surface area The result is a high calcium lime that will provide reliable performance for acid neutralization, fluegas desulfurization, sludge stabilization or other related industrial alkali applicationsQuicklime Graymont2021年12月20日 The Calcium Carbonate Manufacturing Process Natural mineral sources are necessary to make commercially usable calcium carbonate Manufacturing processes are used to improve specific properties and reduce Calcium Carbonate Manufacturing Process and Domestic consumption information identifies consumers of lime, including both calcium oxide (quicklime) and calcium hydroxide (slaked lime) Calcium oxide is the predominant form of lime consumed In 2018, approximately 35% of calcium oxide consumed in the US was used for ferrous and nonferrous metallurgyOxide Supply Chain – Executive Summary US Environmental

Quick Lime Rk Minerals
Quick Lime, or Calcium oxide, is a versatile chemical compound that finds applications in numerous industries due to its chemical properties Here are some key applications : Steel Manufacturing : Quick Lime is critical in the steel industry for purifying steel in the blast furnacePROJECT REPORT ON LIME CALCINATION PLANT Free download as PDF File (pdf), Text File (txt) or read online for free Quick lime which is also known as calcium oxide or burnt lime specifically for chemical industries Quick lime/burnt lime is obtained by calcining pure limestone at high temperature Quick lime is used in mortar and plaster, in glass production and in metal Project Report On Lime Calcination Plant PDF Oxide CalciumProperties of Calcium Oxide • Quick lime is an amorphous white solid with a high melting point of 2600 • In the presence of water, it forms slaked lime This process is called slaking of lime CaO+H2O → Ca • It finds its application in manufacturing of Products Specification QUICK LIME2021年3月3日 Lime products provide a key component for many processes such as purifying water, making sugar, cleaning gases, producing iron, constructing buildings, and treating contaminated land, being also additives for manufacturing paper, glass, pharmaceuticals, and toothpaste Figure 73 shows the main applications of lime Lime is much appreciated in Lime SpringerLink

Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime
2024年9月26日 Learn more about Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime) in detail with notes, formulas, steel and paper manufacturing; it is added to water to produce slaked lime, Ca(OH)$2$, which in turn is sold as a water purification agent, during its refining process, with calcium oxideCalcium Oxide + Water → Calcium Hydroxide (slaked lime) + Heat CaO + H 2 O → Ca(OH) 2 + Heat If you are wondering how to make quicklime at home, then all you need is something made of calcium carbonate, such as a piece of chalk or some seashells Heat this over a flame until it turns red hot, and there, you have just made yourself some What is Quicklime and How is it Made? Science StruckThis process, known as calcination, involves heating the limestone to extremely high temperatures, typically ranging between 1,070°C and 1,270°C, in specialised kilns During the calcination process, the calcium carbonate undergoes a Quicklime • High Purity Quality Products • Buxton In a controlled environment, it is important to slake calcium oxide (quicklime) because it can produce relatively large amounts of heat For water pH management, lime slurry mixes, lime slurry addition, soil regeneration and more, calcium hydroxide or hydrated lime is employed in an already neutralised stateQuicklime Preparation, Properties, and Applications with FAQs
.jpg)
Making Lime
Quicklime When a calcium limestone or chalk rock, that comprises mainly of calcium carbonate (CaCO 3), is heated in a kiln, it changes by a process called calcination into quicklime also known as 'burnt lime' and chemically is mainly calcium oxide (CaO), and the calcination process releases a gas from the rock which is carbon dioxide (CO 2) Hydrated LimeCalcium oxide (also known as quicklime or burnt lime) is a commonly used chemical compound It is a white, caustic, alkaline crystalline solid at room temperature The chemical formula for calcium oxide is @$\begin{align*}CaO\end{align*}@$ Calcium oxide is produced by heating calcium carbonate (also known as limestone) in a process known as calcinationQ: What is calcium oxide or quick lime? CK12 FoundationCalcium oxide (CaO), commonly known as burnt lime, lime or quicklime, is a widely used chemical compound It is a white, caustic and alkaline crystalline solid As a commercial product lime often also contains magnesium oxide, silicon oxide and smaller amounts of Calcium Oxide Manufacturing Process, ApplicationsWhat is Calcium Oxide? Calcium oxide is a white, odorless, and crystalline solid that is highly reactive with water It is made by heating limestone or other calciumcontaining materials at high temperatures, which causes the material to decompose and release carbon dioxide, leaving behind calcium oxide The chemical formula for it is CaOCalcium Oxide Quicklime Mineral and Chemical Exports
.jpg)
What is the Manufacturing Process of Lime?
Preheated limestone is fed into the lime kiln, where it is heated at high temperatures to decompose into calcium oxide (lime) and carbon dioxide There are several types of lime kilns, such as vertical lime kilns like twin shaft lime kilns , annular shaft lime kilns , double beam lime kilns , etc, and rotary lime kilns 2024年2月1日 Lime (CaO), also known as quicklime, is a white, crystalline, alkaline substance produced by heating limestone (calcium carbonate) to high temperatures During that process, carbon dioxide is driven off, leaving calcium oxide (lime) behind Society has made use of lime for centuries Here are just a few examples: In Ancient Rome, lime was used What Is Lime? Lime AssociationQuicklime lumps, also known as burnt lime or calcium oxide (CaO), are produced by heating limestone (calcium carbonate) at a high temperature in a kiln This process removes carbon dioxide and water, leaving behind calcium oxide in the form of lumps or chunksQuick lime lumps Jeya Enterprises2023年10月11日 The chemical formula of lime or quicklime is CaO The chemical name of lime is calcium oxide On the other hand, the chemical formula of limewater is Ca(OH)2 and the chemical name of this substance is calcium hydroxide Properties of Calcium Oxide Quick lime is an amorphous white solid with a high melting point of 2600°Calcium oxide Hebei Yayang Spodumene Co, Ltd

Lime Treated Soil Construction Manual Graymont
However, the use of lime for soil drying, temporary modification, and permanent stabilization is not limited to highway constructionsee Chapter V for more information What is Lime? Lime in the form of quicklime (calcium oxide – CaO), hydrated lime (calcium hydroxide – Ca[OH]2), or lime slurry 1 can be used to treat soilsHigh Calcium Quicklime (Calcium Oxide — CaO) Markets High calcium quicklime is utilized in soil stabilization for highway and building construction, flue gas desulfurization (FGD), steel manufacturing, nonferrous metals processing, paper manufacturing, municipal sanitation and water treatment Customer InformationHigh Calcium Quicklime USLM2024年8月21日 It undergoes a similar process of calcination to produce highquality calcium oxide that is used in the manufacturing process By understanding the significance of each raw material and its role in the manufacturing process, manufacturers can produce calcium carbonate products with the desired characteristics and applicationsThe Ultimate Guide Calcium Carbonate Manufacturing ProcessIndustrial by products: crushed limestone burnt for industrial purposes produces calcium oxide (burnt or quick lime) Stockpiling and wetting up of calcium oxide converts it to calcium hydroxide (hydrated lime or slaked lime) A byproduct from soda ash manufacturing was a hydrated lime marketed as ‘Nutrilime’Lime and lime quality for acid soils AG Excellence

QUICK LIME AND BYPRODUCTS PEC Consulting Group
Sulfate Process The largest application of lime in pulp manufacturing is as a causticizing agent in sulfate (Kraft) plants Wasted sodium carbonate is then reacted with high calcium lime to generate caustic soda for reuse in the process Plants are able to recover about 9098%Burnt lime or Quicklime is an alternate name for the chemical compound calcium oxide Calcium oxide is represented by the chemical formula CaO It exists as a white crystalline solid at standard conditions for temperature and pressure It is inexpensive and abundant Also, quicklime is caustic and alkaline at standard conditionsQuicklime Properties, Uses and Application VedantuProperties of Calcium Oxide • Quick lime is an amorphous white solid with a high melting point of 2600 • In the presence of water, it forms slaked lime This process is called slaking of lime CaO+H2O → Ca • It finds its application in manufacturing of Products Specification QUICK LIMESeamless Integration Collaboration is more than just working side by side; it's about seamless integration and synergy At Lwama SA, we seamlessly integrate our services with your team, creating a harmonious working environment where ideas flow, problems are solved collectively, and decisions are made in unisonLime, calcium oxide, quicklime, burnt lime, lime production, lime

Quick Lime (Lumps) Product CMI Oman Company
Quick lime, also known as calcium oxide, steel manufacturing, metallurgy, and environmental management Its versatility and effectiveness make it a cornerstone in refining, is an Omani company founded in 1977, specializing in highquality limestone products With stateoftheart facilities and a commitment to excellence Instagram X 2019年2月8日 Quicklime (calcium oxide) and slaked lime (calcium hydroxide) are excluded (heading 2522) According to Wikipedia, Calcium Oxide is in manufacturing of cement, paper, and highgrade steel, for medicinal purpose and insecticides Calcium Hydroxide is used in food, paper industry and in water and sewage treatment plantIndustrial grade calcium oxide classifiable under HSN 2825Throughout the manufacturing process, we carefully monitor the process to produce a high calcium quicklime that is highly reactive and has suitable particle surface area The result is a high calcium lime that will provide reliable performance for acid neutralization, fluegas desulfurization, sludge stabilization or other related industrial alkali applicationsQuicklime Graymont2021年12月20日 The Calcium Carbonate Manufacturing Process Natural mineral sources are necessary to make commercially usable calcium carbonate Manufacturing processes are used to improve specific properties and reduce Calcium Carbonate Manufacturing Process and

Oxide Supply Chain – Executive Summary US Environmental
Domestic consumption information identifies consumers of lime, including both calcium oxide (quicklime) and calcium hydroxide (slaked lime) Calcium oxide is the predominant form of lime consumed In 2018, approximately 35% of calcium oxide consumed in the US was used for ferrous and nonferrous metallurgyQuick Lime, or Calcium oxide, is a versatile chemical compound that finds applications in numerous industries due to its chemical properties Here are some key applications : Steel Manufacturing : Quick Lime is critical in the steel industry for purifying steel in the blast furnaceQuick Lime Rk MineralsPROJECT REPORT ON LIME CALCINATION PLANT Free download as PDF File (pdf), Text File (txt) or read online for free Quick lime which is also known as calcium oxide or burnt lime specifically for chemical industries Quick lime/burnt lime is obtained by calcining pure limestone at high temperature Quick lime is used in mortar and plaster, in glass production and in metal Project Report On Lime Calcination Plant PDF Oxide CalciumProperties of Calcium Oxide • Quick lime is an amorphous white solid with a high melting point of 2600 • In the presence of water, it forms slaked lime This process is called slaking of lime CaO+H2O → Ca • It finds its application in manufacturing of Products Specification QUICK LIME
}@~3SRDG`IA1KP_ICWAA.jpg)
Lime SpringerLink
2021年3月3日 Lime products provide a key component for many processes such as purifying water, making sugar, cleaning gases, producing iron, constructing buildings, and treating contaminated land, being also additives for manufacturing paper, glass, pharmaceuticals, and toothpaste Figure 73 shows the main applications of lime Lime is much appreciated in 2024年9月26日 Learn more about Calcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked lime) in detail with notes, formulas, steel and paper manufacturing; it is added to water to produce slaked lime, Ca(OH)$2$, which in turn is sold as a water purification agent, during its refining process, with calcium oxideCalcium Oxide (Quick Lime) and Calcium Hydroxide (Slaked limeCalcium Oxide + Water → Calcium Hydroxide (slaked lime) + Heat CaO + H 2 O → Ca(OH) 2 + Heat If you are wondering how to make quicklime at home, then all you need is something made of calcium carbonate, such as a piece of chalk or some seashells Heat this over a flame until it turns red hot, and there, you have just made yourself some What is Quicklime and How is it Made? Science Struck